Solutions
key notes :
Definition of a Solution:
- A solution is a mixture where one substance (solute) dissolves completely in another (solvent).
Components of a Solution:
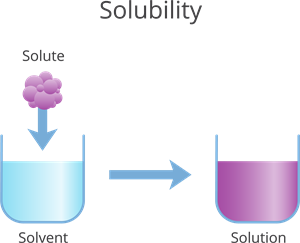
- Solute: The substance that dissolves (e.g., sugar, salt).
- Solvent: The substance in which the solute dissolves (e.g., water).
Examples of Solutions:
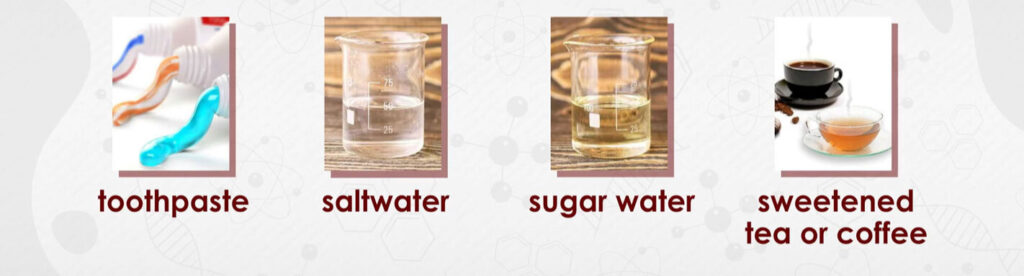
- Sugar dissolved in water.
- Salt dissolved in water.
- Lemonade (lemon juice and sugar mixed in water).
Types of Solutions:
- Liquid solutions: Saltwater, vinegar.
- Solid solutions: Alloys like steel (iron and carbon).
- Gas solutions: Air (a mixture of gases).
Properties of Solutions:
- Solutions are usually clear or transparent (e.g., saltwater).
- The solute does not settle down or separate from the solvent.
- Solutions are uniform (same throughout).
Solubility:
- The ability of a solute to dissolve in a solvent.
- Example: Sugar is soluble in water, but oil is not.
Factors Affecting Solubility:
- Temperature: Hot water dissolves sugar faster than cold water.
- Stirring: Mixing helps solutes dissolve faster.
- Amount of solute and solvent: Too much solute may not dissolve (saturation).
Saturated Solutions:
- A solution that cannot dissolve more solute at a given temperature.
Real-Life Applications:
- Making tea or coffee.
- Preparing saltwater for cooking.
- Mixing medicines.
Experiments to Try:
- Dissolve different substances (e.g., sugar, salt, sand) in water to observe solubility.
- Heat water and check how much more solute dissolves compared to cold water.
Let’s practice!

